History
1,2-Indanediones were first synthesized as intermediates for the preparation of substituted ninhydrins (e.g., 5-methylthioninhydrin) by Prof. Madeleine Joullié and her graduate students, Dr. Diane Hauze and Dr. Olga Petrovskaia, from the University of Pennsylvania (UPenn). During the evaluation of the compounds at the U.S. Secret Service (USSS) by Dr. Tony Cantu and Robert Ramotowski, it was discovered that these compounds were also capable of visualizing latent prints in a manner similar to DFO (producing fluorescence but often weak visibility) [1a-c]. The first article on IND appeared in 1997 [1a].
Over the years many formulations have been proposed [2-7] and varying levels of success reported. Post-treatment with zinc chloride also gave varying results. Australian researchers [9a-c] found around 2005 that humidity had a major effect on the development of fluorescing prints. If the humidity in the lab was below 60% 1,2-IND performed worse than DFO but when humidity was above 70% it gave excellent results [9c]. This cricital value of 70% relative humidity was also observed in the US [10].
They introduced formulations that were less reliant on humidity where a small amount of zinc chloride in ethanol was added to the working solution. They also introduced a method they had already developed for DFO in which the treated papers are heated for a short time (10 seconds) at a much higher temperature (160 °C) than had been used previously for heating in an oven (for example 10 minutes at 100 °C).
When a suitable light source (green light) and filters for a camera are available the number of prints that can be visualized on paper is higher with the use of 1,2-IND than with ninhydrin.
Substituted indandiones
A number of substituted indanediones (e.g., 5-methoxy and 5,6-dimethoxy) gave superior fluorescence compared to DFO (especially the 5,6-dimethoxy-1,2-indanedione [1a-c,2, 3]). Unfortunately, synthesis of these substituted indanediones is somewhat complex and makes their production rather costly. In addition, 5,6-dimethoxy-1,2-indanedione was found to suffer from low solubility in the preferred apolar solvents (e.g., petroleum ether) used in most fingerprint laboratories.
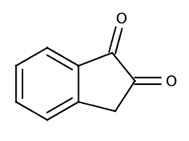 |
|
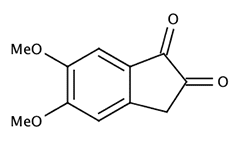 |
| 1,2-indanedione | |
5,6-dimethoxy-1,2-indanedione |
IND versus DFO
Strong latent prints will produce a pink color (Joullié's Pink) with 1,2-IND a bit lighter than with ninhydrin [9b] but weak prints will only be faintly visible or invisible like prints developed with DFO often are. The fluorescence of the prints developed with 1,2-IND however is most of the times stonger than the fluorescence of those developed with DFO.
 |
|
 |
| Fingerprint cut in half and treated with DFO (bottom) and 1,2-IND (top - IND-Zn formulation). | Viewed under green light (505 nm), through an orange filter (OG550). |
The testing and validation of 1,2-indanedione by groups and labs around the world has shown that it can be used as a replacement for DFO and many labs have already adopted it for daily work.
Formulations
Because 1,2-IND is a reasonably soluble compound it doesn't need a large amounts of polar solvents to stay dissolved in mixtures with apolar solvents. Which is a nice property with regard to preventing the running of inks when documents are dipped in the working solution.
The solvents that make up the bulk of the working solutions are either aliphatic solvents like pentane, hexane, or petroleum ether or the fluorinated solvent HFE7100 (1-methoxynonafluorobutane). HFE7100 was found [9b, page 48] to give far better results than HFC4310mee (1,1,1,2,3,4,4,5,5,5-decafluoropentane) for some reason. HFE7100 was preferred above petroleum ether (see thesis Christie Wallace reference 9b, page 48) but research on IND-Zn formulations by researchers at the USSS [10] found them to be equal in performance.
Working solutions with 1,2-IND are not stable indefinitely. Indanedione molecules will eventually interact to form compounds that will not visualize latent prints. Therefore, working solutions should be made up whenever needed and should not be kept for extended periods of time. However, in the powder form the compound is completely stable.
Methanol and ethanol should not be used in working solutions. They were found to be not stable over more than a few weeks [4, 5] which might have to do with the formation of ketals (e.g. 2,2-diethoxy-1-indanone in the case of ethanol). The IND-Zn formulation contains a small amount of ethanol (4 ml per litre) but this apparently has only a small effect on the stability. A shelf life of 3 months for the HFE-7100 working solution when stored in the dark and at room temperature is mentioned [13].
In most formulations acetic acid is included. A notable exception is the formulation published by Wiesner et al. [5] consisting of 2 g/litre IND, 70 ml ethyl acetate and 930 ml of HFE7100 (dissolving the IND in the ethyl acetate and then diluting with HFE7100). They found that including acetic acid in the formulation did not improve results and actually reduced the clarity of the prints. Perhaps this has to do with the paper used in Israel which was either acidic or neutral and the low relative humidity there. In other parts of the world paper is often alkaline (due to the use of calcium carbonate as a filler). On thermal paper the same effect was observed by Chen et al. [15]. Acetic acid diminished fluorescence and blurred the prints when it was included in petroleum ether/ethyl acetate formulations.
The Israeli researchers treated 1000 used checks. Half of them where treated with IND and the other half with DFO followed by ninhydrin. They found that IND developed 50% more prints than DFO alone and 46% more than DFO followed by ninhydrin [5].
This "Wiesner" formulation was evaluated and validated by the FBI and Florida Department of Law Enforcement in 2002 [7].
When prints have been developed with a formulation without zinc chloride weak prints might become stronger fluorescent by post-treatment with zinc chloride solution. Cooling in liquid nitrogen, after zinc treatment, was found by Australian researchers [3] to further increase the fluorescence.
The IND-Zn recipe from Australia has gone through a number of iterations. The first one published in 2007 [9d] used 1 gram of IND dissolved in 30 ml of dichloromethane, 60 ml of ethyl acetate, and 10 ml glacial acetic acid diluted with 900 ml of HFE7100. To this 20 ml of an HFE7100 solution containing 0.04 gram of zinc chloride and 1 ml of ethanol was added to make the working solution (molar ratio IND:ZnCl2 = 23 : 1).
The IND-Zn recipe from 2008 used 0.8 gram of IND dissolved in 90 ml ethyl acetate and 10 ml acetic acid diluted with 900 ml HFE7100 (stock solution) to which 4 ml of a 40 g/liter zinc chloride solution in ethanol was added for the working solution (molar ratio IND:ZnCl2 = 4.7 : 1).
The IND-Zn recipe published in the NCFS workshop manual [13] is shown below. The molar ratio IND:ZnCl2 = 3.5 : 1 in this recipe.
| Australian IND-Zn working solution, 1 litre | Zinc chloride stock solution, 50 ml |
| 0.6 g 1,2-Indanedione dissolve in 125 ml Ethyl acetate, then add 5.2 ml acetic acid (99%), then add 870 ml HFE-7100, then add 4 ml Zinc chloride stock solution |
2.0 g Zinc chloride dissolved in 50 ml Absolute Ethanol Shelf life is indefinite. ZnCl2 concentration = 0.04 g/ml |
Dana Bicknell and Robert Ramotowski of the USSS [10] took the first recipe of Stoilovic et al. [9d] and varied the amounts of the zinc chloride solution added to the stock solution. They concluded that a four-fold increase of the amount of zinc chloride added (0.16 g/l; molar ratio IND:ZnCl2 = 5.75 : 1) gave them the best results. They also found that there was no difference between the performance of the solution made with HFE7100 or petroleum ether and opted for the latter because of cost.
A formulation without zinc chloride based on a recipe developed by the PSDB [6] which compared favorably with other known formulations [9b] is shown below. The change to the recipe is an increase in the concentration of IND (from 0.25 to 1 g) and exchanging HFE7100 for petroleum ether.
| Adapted PSDB formulation, 1 litre |
| 1 g 1,2-indanedione 10 ml glacial acetic acid (99-100%) 90 ml ethyl acetate 900 ml petroleumether |
For all IND formulations it is recommended to store the solution in a dark brown glass bottle, in the dark. From the chemical literature it is known that 1,2-indanediones are prone to photochemical reactions upon exposure to sunlight.
Developing and viewing the prints
The best method for developing prints on documents treated with 1,2-indanedione appears to be heating for a short time (10 seconds) on a temperature of 160 °C, using a heat press or something of that nature (Kasper et al. used a photographic mounting-press at 100 °C).
Heating papers in an oven (100 °C, 20 minutes) will develop prints but the best results appear to be obtained with the fast and short heating at a high temperature. In experiments where IND and amino acids were reacted in solvents it appeared that when the reaction goes slowly or for an extended period of time (non-fluorescent) products are formed by reaction of the Joullié's Pink with unreacted IND molecules to form brown colored oligomers [9b, section 5.3.3].
Prints developed with 1,2-indanedione will fluoresce under green light (optimum about 530 nm), similar to DFO. Optimum viewing and photographing is done with dark orange glasses/filters (cut on point 570-590 nm).
Developing prints on low-quality paper
On a number of low quality papers (like newspapers, cardboard, and recycled paper in general) we found that indanediones did not develop fingerprints, where ninhydrin and DFO did. In general, however, the UK Police Scientific Development Branch (PSDB) concluded after experiments with a range of papers that in practice there is little difference between DFO and 1,2-indanedione. On brown envelopes they developed more prints with 1,2-IND than with DFO [14, Chapter 5, p. 384].
Developing prints on these kind of papers is problematic in general. The reason for this is still unknown.
Developing prints on thermal paper
Thermal paper is a heat sensitive paper that in the past was used almost exclusively for fax paper. Nowadays, it is used in many applications like receipts from automated teller machines (ATMs), point-of-sale registers, and parking payment machines. The active layer in the paper is very susceptible (turns dark or the print dissolves) for polar solvents like acetone, ethanol and acetic acid and of course heat. This makes developing prints on these papers problematic.
Jon Stimac of the Oregon State Police was the first to publish on the use of IND on thermal paper [8]. He showed that the HFE-7100 formulation published by Wiesner et al. [5] can be used on thermal papers.
The formulation using an indandione-zinc recipe from the Australian Federal Police (Stoilovic et al. [13]) is shown below.
| Thermal IND-Zn working solution, 1 litre | Zinc Chloride stock solution, 50 ml |
| 0.35 g 1,2-Indanedione dissolve in 40 ml Ethyl acetate, then add 960 ml HFE-7100, then add 4 ml Zinc chloride stock solution |
2.0 g Zinc chloride dissolved in 50 ml Absolute Ethanol Shelf life is indefinite. ZnCl2 concentration = 0.04 g/ml |
Sensitivity to light
Research has shown that developed prints are sensitive to daylight. Decreasing fluorescence intensity was observed when prints were not stored in the dark. When stored in the dark, prints retain their fluorescent properties for a long time.
The green light used to view and photograph the fluorescence appears to cause only a temporary decrease in fluorescence intensity, and only after extended periods of exposure (several hours). The fluorescence returned after storage in the dark overnight, according to the PSDB.
Procedure for preparation of the zinc chloride solution
For the treatment with zinc chloride, we developed the following non-CFC formulation. We have found it to be very reliable and stable (we have stored and used solutions that were several years old, without any problem).
Dissolve 30 grams of zinc chloride in a mixture of 500 ml methyl-tert-butylether (MTBE) and 20 ml of anhydrous ethanol (98% or more) using a 1 liter erlenmeyer flask, a magnetic stirring bar, and a magnetic stirrer, in a fume hood. It will take about 30 to 60 minutes to dissolve completely.
After it is fully dissolved, add 10 to 20 ml of glacial acetic acid (99-100%) and dilute with 500 ml of a hydrocarbon solvent (petroleum ether, pentane, heptane). Store in a brown glass bottle.
The zinc chloride solution is particularly stable, also at low temperatures. In principle it can be kept indefinitely.
Procedure for treatment with zinc chloride
Zinc chloride treatment of prints on paper, developed with 1,2-indanedione (using a formulation other than a IND-zinc one) is a very simple procedure. We recommend that a sprayer should be used in a fume hood to apply the zinc chloride. Spray the solution on the article lightly, allow the solvents to evaporate, and then spray the article again if needed. There is, however, no change of color or any other indication that spraying was sufficient as in the case of ninhydrin.
Optimum results are obtained with heating the paper again after the zinc chloride treatment for 10 seconds at 160 °C [9d].
References
[1a] Ramotowski, R.; Cantu, A.A.; Joullié,
M.M.; Petrovskaia, O., "1,2-Indanediones: A Preliminary Evaluation of a New
Class of Amino Acid Visualizing Compounds", Fingerprint Whorld 1997,
Vol. 23, No. 90, pp. 131-140.
[1b] Joullié, M.M.; Petrovskaia, O., "A better way to develop fingerprints",
CHEMTECH 1998, Vol. 28, No. 8, pp. 41-44.
[1c] Hauze, D.; Petrovskaia, O.; Taylor, B.; Joullié, M.; Ramotowski, R.; Cantu, A., "1,2-Indanediones: New Reagents for Visualizing the Amino Acid Components of Latent Prints," Journal of Forensic Sciences 1998, Vol. 43, No. 4, pp. 744-747, [doi:10.1520/JFS14300J].
[2] Almog, J.; Springer, E.; Wiesner, S.; Frank, A. et al., "Latent
Fingerprint Visualization by 1,2-Indanedione and Related Compounds: Preliminary
Results", Journal of Forensic Sciences 1999, Vol. 44,
No. 1, pp. 114-118, [doi:10.1520/JFS14421J]
[3] Roux, C.; Jones, N.; Lennard, C.; Stoilovic, M., "Evaluation of
1,2-Indanedione and 5,6-Dimethoxy-1,2-Indanedione for the Detection of Latent
Fingerprints on Porous Surfaces", Journal of Forensic Sciences 2000,
Vol. 45, No. 4, pp. 761-769, [doi:10.1520/JFS14768J]
[4] Unpublished observations by researchers from the University of Pennsylvania,
USSS, Israel National Police, and PSDB.
[5] Wiesner, S.; Springer, E.; Sasson, Y.; Almog, J., "Chemical Development of Latent Fingerprints: 1,2-Indanedione Has Come of Age", Journal of Forensic Sciences 2001, Vol. 46, No. 5, pp. 1082-1084, [doi:10.1520/JFS15102J]
[6] Merrick, S., Gardner, S.J.; Sears, V.G.; Hewlett, D.F., "An Operational Trial of Ozone-Friendly DFO and 1,2-Indanedione Formulations for Latent Fingerprint Detection", Journal of Forensic Identification 2002, Vol. 52, No. 5, pp. 595-605.
[7] Kasper, S.P.; Minnillo; D.J., Rockhold; A.M., "Validating IND (1,2 - indanedione)" Forensic Science Communications 2002, Vol. 4, No. 4.
[8] Stimac, J.T. "Thermal Paper: Latent Friction Ridge Development via 1,2-Indanedione" Journal of Forensic Identification 2003, Vol. 53, No. 3, pp. 265-271.
[9a] Wallace, Christie, "Characterisation and Optimisation of Reaction of 1,2-Indanedione with Amino Acids and its Application to Fingerprint Casework", presentation at the 17th International Symposium on the Forensic Sciences, March 2004 (abstract - from web.archive.org, the original site www.anzfss2004.org.nz doesn't exist anymore).
[9b] Wallace-Kunkel, Christie; "Evaluation of Reagents for the Chemical Enhancement of Fingermarks on Porous Surfaces: Optimisation and Characterisation of the 1,2-Indanedione Technique", Thesis, University of Technology Sydney, 2008 [hdl.handle.net/10453/32042]
[9c] Wallace-Kunkel, Christie; Lennard, Chris; Stoilovic, Milutin; Roux, Claude, "Optimisation and evaluation of 1,2-indanedione for use as a fingermark reagent and its application to real samples" Forensic Science International, 2007, Vol. 168, No. 1, pp. 14-26 [doi:10.1016/j.forsciint.2006.06.006]
[9d] Stoilovic, Milutin; Lennard, Chris; Wallace-Kunkel, Christie; Roux, Claude, "Evaluation of 1,2-Indanedione Formulation containing Zinc Chloride for Improved Fingermark Detection on Paper" Journal of Forensic Identification 2007, Vol. 57, No. 1, pp. 4-18.
[10] Bicknell, D.E; Ramotowski, R., Journal of Forensic Sciences 2008, Vol. 53, No. 5, pp. 1108-1116.[doi:10.1111/j.1556-4029.2008.00826.x]
[11] Stoilovic, Milutin, Instructions for preparation and use of the IND-Zn reagent (personal communication, 2008).
[12] Spindler, X.; Shimmona, R.; Roux, C.; Lennard, C. "The effect of zinc chloride, humidity and the substrate on the reaction of 1,2-indanedione-zinc with amino acids in latent fingermark secretions", Forensic Science International, 2011, Vol. 212, pp. 150-157 [doi:10.1016/j.forsciint.2011.06.005].
[13] Stoilovic, M., Lennard, C. NCFS Workshop Manual: Fingermark Detection & Enhancement. 6th ed. National Centre for Forensic Studies, Canberra; 2012.
[14] Fingerprint Source Book, published March 2013 (v 1.0), CAST/Home Office
[15] Chen, C.; Yu, Y.; Lee, H.C.; Giang Y., Wang, S. "Latent Fingerprint Development on Thermal Paper Using Traditional Ninhydrin and 1,2-indanedione" Journal of Forensic Sciences 2016, Vol. 61, No. 1, pp. 219-225 [doi:10.1111/1556-4029.12897]












