Introduction
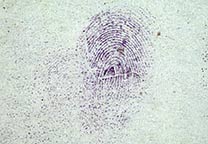
Ninhydrin is the most well-known and most used reagent for the development of fingerprints on paper and other porous materials (like carton, latex painted walls, wallpaper etc.). Ninhydrin (1,2,3-indantrione, mono hydrate) reacts with amino acids in sweat, deposited as a fingerprint, forming a very strongly colored compound, called Ruhemann’s purple.
Despite the fact that there is only a minute amount of material in a fingerprint, the strong color of the Ruhemann’s purple makes it possible that the fingerprint becomes visible.
Alternative reagents used instead of ninhydrin are 5-MTN (a ninhydrin analog), DFO, and 1,2-IND. These reagents are able to develop fluorescent products with amino acids and reveal more fingerprints when there is a possibility to photograph this fluorescence (using alternate light sources and colour filters in front of the camera lens).
Ninhydrin formulations
Ninhydrin was synthesized for the first time in 1910 by Siegfried Ruhemann (an English chemist) and soon after that used as an analytical reagent for amino acids. It was not until the nineteen fifties that its use a reagent for the development of fingerprints was described by two Swedish chemists (Odén and von Hofsten [1a]). They used ninhydrin dissolved in ether (diethylether) or acetone with or without the addition of acetic acid. Odén patented the use of ninhydrin and the use of a copper salt for fixing the prints in Great Britain and the USA [1b, 1c] despite the fact that it was widely known that in paper chromatography of amino acids and subsequent treatment with ninhydrin fingerprints would show when one wasn't careful in handling the paper.
Ether is a rather dangerous solvent due to its low boiling point (so strong evaporation at room temperature) and high inflammability. Ninhydrin dissolves well in acetone, ballpoint inks however also.
An improved formulation (less ink-running) based on petroleum ether was published by D.A. Crown in 1969 [3]. Addition of 1% acetic acid to this formulation improved the sensitivity [2].
A noticeable improvement to the formulation initiated by the Home Office Police Scientific Development Branch (now called CAST) was the introduction of 1,1,2-trichlorotrifluoroethane (also known as Fluorisol, CFC113, and frigen) as the bulk solvent in 1974 (Morris and Goode, [2].) This formulation (called NFN) had good sensitivity, low ink-running properties, low flammability, and evaporated quickly. Due to the ozone depletion potential of chlorofluorocarbons it's use got banned in the early 1990s.
The NFN reagent was made by combining 30 ml of a stock solution (4.5 gram of ninhydrin crystals, 9 ml of glacial acetic acid, 18 ml absolute ethanol [2b]) with 1 liter of CFC113 (preferably dried on molecular sieves type 3A). Replacing the CFC113 by petroleum ether (a mixture of hydrocarbons like hexanes and heptanes) in this formulation is not possible. The stock solution will not or only partially dissolve in the petroleum ether and will give a two-phase solution. A thin yellow layer of stock solution will be on the bottom, the colorless petroleum ether will float on top.
When CFC113 got banned we developed our own formulation, adding an ether (methyl-tert-butylether, MTBE) as the main change to the formulation to keep the ninhydrin solution as one phase, uniform solution.
Other formulations are known for example one in which more ethanol is used. This has the disadvantage of increasing the ink-running.
A good formulation on the basis of heptane was published by Hewlett and Sears (from the Home Office Police Scientific Development Branch) in 1997 [4]. It combines 5 gram of ninhydrin crystals, 3 ml acetic acid, 75 ml ethanol, and 25 ml ethyl acetate for a concentrated solution that is diluted with 1 liter of heptane to obtain the working solution. Compared to the NFN-formulation the amount of ethanol is increased 4 times and the amount of acetic acid slashed by two-thirds, 25 ml of ethyl acetate is added. The performance in number of fingerprints developed on checks was better than the NFN-formulation and the formulation based on HFE7100 published at the same time [5a] by the authors [4,5a]. Due to the flammability and associated risks of explosions for example in ovens when papers where not dry enough, it was not recommended for use by British police forces. Instead the HFE7100-formulation [5a] was recommended. The costs of HFE7100 are appreciably higher than petroleum ether or even heptane though.
In our view working in a fume hood is necessary for both the heptane/petroleum ether and HFE7100 based formulations so equipment costs are the same. When papers (and especially items like corrugated carton boxes) are allowed to dry for a sufficient time before heating them in an oven there is little to no risk of explosion.
Especially for thermal paper (cash receipts for example) there is a ninhydrin derivative called ThermaNin. This is a hemiketal of ninhydrin and a long-chain alcohol. Applied to paper it will react with the water present and falls apart in ninhydrin and the alcohol, leaving the ninhydrin free to react with the fingerprints.
Treating papers with working solution
The treatment of papers with the petroleum ether based ninhydrin working solution is not complicated. However, due to the evaporation of the petroleumether which fumes can form an explosive mixture with air there is a need to work in a fume hood.
To treat documents or other papers with ninhydrin a shallow dish can be used in which a thin layer of working solution is poured (about 1-2 cm deep). Either put the paper in the solution or draw it through. It should not be longer in the solution than about 5 seconds, just complete wetting of the paper is sufficient.
For the treatment BVDA has a practical plastic dipping-tray in the
program. Because of the round shape of the bottom one can work with
small amounts of working solution which results in less unwanted
evaporation. Complete wetting of the paper is sufficient for treatment.
Use tweezers (preferably of a kind that has no grooved surfaces) to draw
the paper through the solution.
Large surfaces (like carton boxes for example) or paper labels of bottles can also be treated using a soft brush.
Small amounts of used working solution can better be discarded as chemical waste (non-halogenated organic solvents). Larger amounts that are not visibly contaminated can be saved for later use. Do not add it back to a container with fresh solution, store it separately in for example a brown glass bottle (do not forget to label it, with the date of use.)
When the solution is fresh it is light yellow. When due to contamination with water, for example through condensation or from water in the treated paper, a separation of concentrated ninhydrin solution and petroleum ether occurs, this is evident from the presence of the concentrated solution at the bottom and the colorless solution above it.
Before papers (especially corrugated cardboard) are put in an oven to accelerate the development of the fingerprints the solvents in the working solution must have evaporated completely.
DFO, IND, and ninhydrin
Ninhydrin can be used after papers have been treated with DFO first
(it is even recommended to do this after DFO treatment, when development
of fingerprints and photography of prints with sufficient detail has
been done) but in principle DFO cannot be used after treatment with
ninhydrin.
Reportedly, there have been cases where a fingerprint became visible
after treatment with DFO, that had not been visible after prior
treatment with ninhydrin.
Applying ninhydrin after a paper has been treated with 1,2-IND does not yield any additional prints.
Ninhydrin spray
A very user-friendly and quick method to treat objects or papers with ninhydrin is the use of ninhydrin in an aerosol can. Ninprint was developed by us because existing sprays either contained CFC’s or contained too much polar solvents to our liking. An excess of polar solvents causes ink-running. Ninprint does not contain acetic acid, due to its corrosive nature. The Ninprint spray is very "dry" because the solvents evaporate very quickly. The advantage of this is that inks hardly get the chance to run. It does mean that a piece of paper is not fully wetted with the spray, so both sides of the paper need to be sprayed.
The spray mist of the Ninprint is quite irritant for the airways. Probably, this is caused by the presence of small ninhydrin crystals due to evaporation of the solvents. Use it in a fume hood, outside or with a very tight mask (take care of eye-protection too, wear safety glasses).
Developing the prints
Fingerprints become visible through the reaction of proteins and amino acid present in the sweat with which they were deposited on the surface and ninhydrin. The reaction produces a purple compound called Ruhemann’s purple.
A very dependable, but slow, method to develop fingerprints after treatment with ninhydrin is to store treated papers in the dark and elevated relative humidity (80%). This can be accomplished for example by storing the papers in a big plastic bag in which a dish with moist paper or cotton balls is put. Direct contact between the wet paper or cotton and the treated papers should be avoided at all costs.
A method to create a humidity of about 80% in a not too large space is to place a large dish with a saturated table salt (NaCl) solution with a layer of salt [7a] on the bottom.
Full development at room temperature can take several days to weeks.
Accelerated development
The development of fingerprints can be accelerated by heating the treated papers for some time at a higher temperature (and ideally also an elevateded humidity) in an oven. Temperatures above 80 °C are not advised. The maximum heating period at this temperature is 5 minutes. At lower temperatures this time can be longer. Obviously, the object should be able to stand these temperatures. Plastic bottles with a paper label will probably still tolerate a temperature of 50 °C. In case of doubt do a test with a similar article.
The background coloration will be stronger with the application of heat than with development at room temperature. For maximum contrast development at room temperature (or higher temperature using a special climate cabinet with raised humidity like a NINcha chamber) is advised.
After the heat treatment the object should be stored in the dark, at room temperature and if possible elevated humidity for further development (at least 10 days if possible). Many fingerprints will only become visible after several days.
A method used in some laboratories is using a steam iron instead of an oven. The hot iron is moved over the surface of the paper at a distance of about 1.5 cm. The iron should not touch the paper! On carton or coated paper this method is not advised, since the steam has the tendency to condense on these surfaces. This causes damage to or destruction of the fingerprints.
Changing color (toning) with metal salts
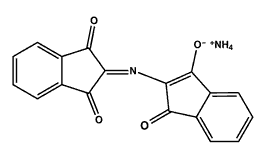
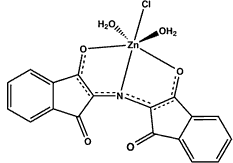
It is known that the Ruhemann's purple can complex with metal salts like nickel nitrate, cadmium and zinc chloride leading to a color change (nickel and cadmium give a red color, zinc usually an orange).
Using cadmium is not advisable for safety and environmental reasons, because cadmium salts are highly poisonous. The cadmium and zinc complexes of Ruhemann’s purple can also fluoresce. In general cooling with liquid nitrogen (temperature of -196° C) is necessary to get sufficient fluorescence using green light (fluorescence is in the red region) but sometimes also fluorescence is seen at room temperature. The fluorescence is less intense than that of DFO.
The process itself is not very complex. In practice whether zinc treated prints fluoresce or not has been shown to depend strongly on the conditions used for developing the ninhydrin treated prints, the type of paper, and unknown factors. The prints should not have been subjected to heating (at low humidity) for accelerating the development and post-processing with zinc should be done fairly soon after ninhydrin treatment. A succesful outcome is thus not assured. See for more information and literature citations Wainwright's et al. article [8].
By modifying the ninhydrin molecule (for example attaching a methoxy or a methylthio substituent [5-MTN] to the aromatic ring) it was found that both the reliability and intensity of the fluorescence is significantly better.
Changing the color with a zinc salt (also known as zinc toning) can be useful in absorption mode also. When viewing or photographing the orange colored prints under blue light a better contrast is obtained than with the purple colored print with white (or any other color) light. Especially when the paper contains blue or blue-green printed areas this method is very useful. Blue and blue-green printed areas lose contrast with this type of lighting [9].
Example of zinc chloride treatment of a ninhydrin developed print
Treatment with zinc chloride
Zinc chloride treatment of prints on paper, developed with ninhydrin, is a very simple procedure. BVDA has developed a zinc chloride solution, on a MTBE/petroleum ether basis. This contains a high concentration of zinc chloride (30 grams/liter), so that only small amounts are needed.
It is possible to dip the paper in the zinc chloride solution. However, it has the potential to run inks and the concentration of zinc in the solution is higher than needed for dipping.
We recommend that a sprayer should be used in a fume hood to apply the zinc chloride. Spray the solution on the article only lightly and allow the solvents to evaporate. For the formation of the fluorescing complex a small amount of water is needed. Usually, enough water is present in the air for this to happen. The complexation will normally take only a few minutes. Humidifying a print (by breathing on it) speeds up the process. Only if the color change is not complete, spray again.
By spraying the paper with zinc chloride solution, accurate control over the dosing is obtained. Also running of ballpoint inks can be prevented best this way.
Safety
Always use well-fitting gloves, safety glasses and protective
clothing like a lab coat when using ninhydrin solutions or sprays. Avoid
contact of the skin with ninhydrin solutions (it will stain the skin
purple). If it does happen inadvertently, rinse the skin with cold
water. If the ninhydrin solution gets in the eyes, rinse them thoroughly
with running water.
In case irritation or other symptoms (apart from staining of the skin, that will last a few days) persist consult a physician.
It is strongly advised to only use ninhydrin solutions in a fume hood. If that is not possible (for example when treating wall paper in a room) good ventilation is a necessity. Petroleum ether is very volatile and in small rooms will displace the air. The vapor/air mixture is explosive in a certain concentration range.
If HFE7100 is used as a solvent it is important to remember that it is not retained by activated carbon filters as used in recirculating fume cupboards [11].
Ninhydrin will strongly color the skin. As a powder it irritates the airways. Because after the solution has evaporated small ninhydrin crystals can have formed on the paper it is advised to store the papers after treatment and development in plastic bags (for example zip-lock bags). This will also minimize the chance that new prints are made on the papers, but still allows visual inspection. Note that polyethylene bags will allow some of the ninhydrin to escape. Polypropylene bags are not permeable for ninhydrin [12].
References and footnotes
[1a] Odén, S.; Hofsten, B. von "Detection of Fingerprints by the
Ninhydrin Reaction" Nature, 1954, 173, p. 449-450. (PDF)
[1b] Odén, Svante, "A process of Developing Fingerprints on paper and the like materials.", Patent GB767341, published January 30, 1957. Downloaded from the EPO website.
[1c] Odén, Svante, United States Patent 2,715,571, August 16, 1955. Downloaded from the Google website
[2a] Morris, J.R.; Goode, G.C. Police Research Bulletin, No. 24, 1974, p. 45-53. (PDF)
[2b] The NFN ("new formulation for the ninhydrin reagent") formulation uses a stock solution
made from 25 gram ninhydrin crystals, 50 ml of glacial acetic acid and
100 ml of absolute ethanol (98% or better) and combining 30 ml of this
stock solution with 1 liter of 1,1,2-trichlorotrifluoroethane. In
practice this amounts to about 4.5 gram ninhydrin crystals, 9 ml acetic
acid, 18 ml of ethanol in 1 liter of Fluorisol. Due to the increase in
volume caused by the relatively large amount of ninhydrin in the stock
solution the volume of it is larger than 150 ml (5x30).
[3] Crown, D.A. "The Development of Latent Fingerprints with Ninhydrin", Journal of Criminal Law, Criminology & Police Science, 1969, Vol. 60, No. 2, p. 258. DOI: 10.2307/1142254
[4] Hewlett, D.F.; Sears, V.G. "Replacements for CFC113 in the Ninhydrin Process, Part 1", Journal of Forensic Identification 1997,
Vol. 47, No. 3, p. 287-299.
[5a] Hewlett, D.F.; Sears, V.G.; Suzuki, S. "Replacements for CFC113 in the
Ninhydrin Process, Part 2", Journal of Forensic Identification
1997, Vol. 47, No. 3, p. 300-306.
[5b] The HFE7100-formulation is made from 5 gram ninhydrin crystals, 5
ml acetic acid, 45 ml ethanol, and 2 ml ethyl acetate to 1 liter of
HFE7100.
[6] Mixture of the hydrofluoroethers (HFE's)
1-methoxynonafluoroisobutane and 1-methoxynonafluorobutane, CAS-number
163702-08-7 and 163702-08-6 respectively, marketed by 3M till 2026 under the brand name NovecTM Engineering Fluid HFE-7100.
[7a] This solution is made by dissolving as much (table) salt in (tap)
water till no more dissolves (about 300 grams per liter). To be sure
that the solution is saturated, part of the salt added should not
dissolve. The mixture of salt and saturated solution can then be
transferred to the dish.
In a glass container the salt solution tends to creep up and over the
walls after a while. Using a PVC container or a Goretex membrane as
described in a newsletter of the Seattle Art museum might prevent that practical issue.
[7b] Nielson, J.P., "Quality Control for Amino Acid Visualization Reagents", Journal of Forensic Sciences, 1987, Vol. 32, No. 2, p. 370-376, [doi:10.1520/JFS11140J]
for practical examples of fingerprints that do not develop any more at low air humidity after ninhydrin treatment.
[8] Davies, P.J.; Kobus, H.J.; Taylor, M.R.; Wainwright, K.P. "Synthesis and Structure of the Zinc(II) and Cadmium(II) Complexes Produced in the Photoluminescent Enhancement of Ninhydrin Developed Fingerprints Using Group 12 Metal Salts", Journal of Forensic Sciences, 1995, Vol. 40, nr. 4 (July 1995), p. 565-569. DOI: 10.1520/JFS13826JQuote from the Nielson article
Figure 4 depicts four strips which were treated simultaneously with the same batch of ninhydrin reagent; only environmental factors during development were varied. Strips a and d were placed in an environment of 25°C at 60% RH for 18 h before treatment. Strips b and c were placed in a desiccator over Drierite® (CaSO4) for 18 h before treatment. All strips were treated at the same time by dipping them in a 1% ninhydrin: Freon 113 solution [10]. After being processed with ninhydrin, Strips a and c were placed in a chamber at 30°C, 75% RH for 24 h. Strips b and d were placed in a desiccator over Drierite. After 24 h, the strips were evaluated: a and c showed strong (normal) development. Strip b shows barely perceptible development of dilution 0 and d shows perceptible development to Dilution 2.
These results seems to indicate that pretreatment temperature/humidity conditions do not have a great effect on the strength of development (at least over short periods of time). Posttreatment temperature/humidity conditions, however, appear to be critical. A decrease of X1000 in sensitivity between a (or c) and b and d is seen here. The difference in development between b and d (d showing greater sensitivity) is attributed to the fact that some residual humidity was present in the paper immediatly after processing which allowed development to commence before that moisture was removed by the desiccant.
Although relative humidities appoaching zero are not encountered in the environment, very low humidities are routinely encountered in arid or semi-arid climates, and humidities low enough to interfere with optimum development occur in almost any climate at some time. The degree of interference is unknown without a known and replicable standard against which to measure.
Figure 5 depicts four strips which were treated with the same batch of ninhydrin reagent (1%) on succesive days in February and allowed to develop in ambient laboratory conditions. The building housing the laboratory was at that time less than one year old and contained up-to-date environmental controls which supposedly maintain constant humidity. No record of the ambient humidity was kept. Strips were treated in the order a, b, c, d. Of interest is the observation that development appears to be arrested even when a subsequent strip developed more strongly (b, compare c); however, the phenomena has been observed on more than one occasion. In any case, such variability in the intensity of development is common from October to April in temperate climates, when relative humidities may be low unless a controlled temperature and humidity environment is available.
[9] Velders, M.J.M. "Latent Fingerprint Pencil", TIB, 1990, No. 3, p. 7-18.
[10] Fingerprint Source Book Chapter 3, sections 1 to 3.
[11] Fingermark Visualisation Manual, Chapter 6.1. ISBN: 978-1-78246-234-7, Home Office, January 2014.
[12] Schwarz, L.; Hermanowski, M.; "Untersuchung zur Ninhydrinpermeabilität von Verpackungen in der daktyloskopischen Spurensicherung", Archiv für Kriminologie, 2008, No. 222 (5/2008), p. 14-22.
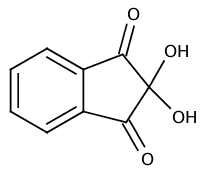
Name: Ninhydrin (2,2-dihydroxyindan-1,3-dione)
CAS-No.: 485-47-2
Pale yellow crystals
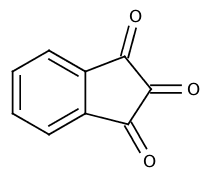
Name: indan-1,2,3-trione
CAS-No.: 938-24-9
Red compound, obtained by heating ninhydrin in vacuum.
Reaction of ninhydrin to Ruhemann's purple
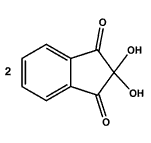 | 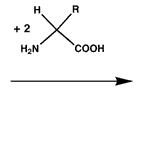 |  |
| 2 x ninhydrin | 2 x amino acid | 1 x Ruhemann's purple |
Complexation of Ruhemann's purple with zinc salt
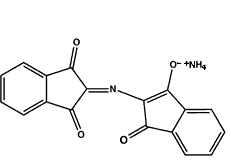 |
 |
 |
| Ruhemann's purple | Zinc chloride | Complex of Ruhemann's purple with zinc ion |