Leuco Crystal Violet (LCV) for staining of marks in blood
Leuco Crystal Violet (LCV) is a reagent for the detection and staining of marks in blood. It is a colorless to light-blue water-based (400 ml in a brown glass bottle, solution A) that is mixed just before use with the included 15% hydrogen peroxide solution (100 ml in a brown plastic bottle). The mixing ratio is 4 parts solution A to 1 part peroxide solution (B).
The reagent is sprayed over the surface where marks in blood are suspected. When the (mixed) reagent comes in contact with blood the hydrogen peroxide is broken down by the hemoglobine. In turn the oxidized hemoglobin oxidizes the the colorless LCV to strongly purple-colored crystal violet. The hemoglobin is now back in it's original state and can be oxidized by hydrogen peroxide again. This is therefore a catalytic reaction (the hemoglobin is the catalyst).
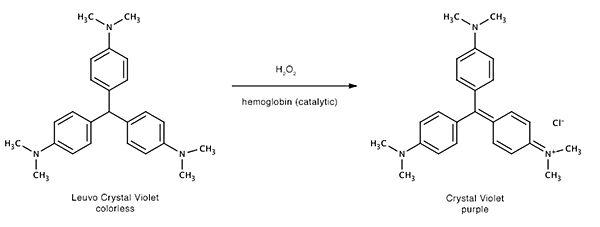
Crystal violet is also known as gentian violet and Basic Violet 3. On non-porous surfaces rinsing with demineralized water to remove excess reagent (which will slowly become purple due to oxidation by air helped by light of the unreacted LCV) is an option.
Once the oxidation to crystal violet has occurred, removing the purple stain is often cumbersome if not impossible. This should be kept in mind when using it on crime scenes.
Advantages of the reagent are:
- in principle there is no need for rinsing which makes it less labor intensive than for instance dye solutions like Amido Black and Hungarian red and makes it possible to use it on materials that do not lend themselves to rinsing like carpet.
- it can be used both on porous and non-porous surfaces.
- the marks do not need prior fixing because the reagent already contains fixative (the working solution contains 2% sulfosalicylic acid).
- it does not contain volatile solvents and with respect to chemical composition not dangerous.
After the application of LCV protein stains like Amido Black and Acid Violet 17 can still be used. Apparently, LCV can also be used after luminol [1], however it first needs fixing with 2% sulfosalicylic acid otherwise leaching with loss of detail occurs.
Under the influence of light unreacted LCV can still be oxidised causing background coloration. This process is however very slow so there is plenty of time to photographically record visualized marks.
Crystal violet is known [2] to fluoresce in the deep-red and infrared after oxidation (especially at low concentrations.) This might yield additional marks or make it possible to enhance developled marks.
Shelf life
Once solution A and B are mixed the shelf life when stored in the dark and cold (refrigerator) is several months. To maximize shelf life BVDA ships the reagent as two separate solutions, one of which contains the hydrogen peroxide. This extends the shelf life appreciably although an exact time is hard to name. It might also depend on the particular batch of LCV and/or other small variations in the chemicals used. On standing the LCV solution slowly darkens. When the surface already becomes purple when there is no blood present, the reagent should obviously be discarted. The hydrogen peroxide solution also slowly decomposes.
Therefore, we strongly recommend to store the reagent in the refrigerator.
History
LCV was conceived and over the course of several years improved by John F. Fischer, who worked for the Orange County Sheriff's Office in Orlando (Florida). The FBI lab in Washington started using it at the end of 1993.
In June 1994 John Fisher gave a presentation on LCV at the FBI academy in Quantico (International Symposium on Footwear and Tire Tread Impression Evidence). Bill Bodziak who at the time still worked for the FBI gave a lecture on it at the first SPTM-conference (the working group later became part of ENFSI) in Helsinki (Finland), May 1995 [1].
In 1996 Bill Bodziak published an article on LCV in the journal Forensic Science International [3].
Footnotes
[1] Handout of a lecture by Bill Bodziak: "The use of Leuco Crystal Violet to enhance shoe prints in blood", Shoe Print/Tool Mark Examiners (SPTM) conference, May 1995, Helsinki (Finland).
[2] Bramble, K.; Cantu, A.A.; Ramotowski, R.R.; Brennan, J.S., "Deep Red to Near Infrared (NIR) Fluorescence of Gentian Violet-treated Latent Prints", Journal of Forensic Identification 2000, Vol. 50, no. 1, pp. 33-49.
Also see the letter by Menzel, E. Journal of Forensic Identification 2000, Vol. 50, nr. 3, blz. 245-249.
[3] Bodziak, William J. "Use of leuco crystal violet to enhance shoeprints in blood", Forens. Sci. Int., 1996, Vol. 82, pp. 45-52 DOI: 10.1016/0379-0738(96)01965-2









